Hampton research代理 HR2-801 TCEP hydrochloride
TCEP hydrochloride
APPLICATIONS
- Stable reducing agent for crystallization trials
FEATURES
- Highly soluble
- Odorless and non-volatile
- Effective at acidic and alkaline pH
DESCRIPTION
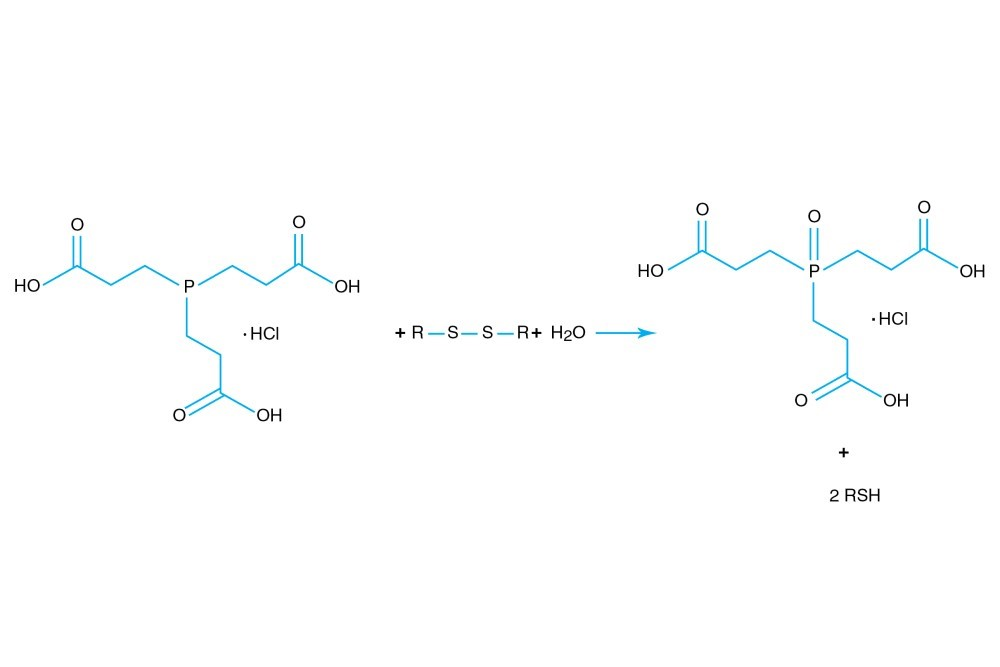
TCEP hydrochloride is Tri(2-carboxyethyl)phosphine hydro-chloride.
TCEP hydrochloride is an odorless, non-volatile reducing agent that is more stable and effective than DTT or 2-Mercaptoethanol. Unlike DTT, TCEP hydrochloride retains its reducing power at acid pHs (pH 5) and at pHs above 7.5 and is stable in air. TCEP hydrochloride is soluble in water to 310 grams per liter. For most applications, 5-50 mM TCEP provides sufficient molar excess to effectively reduce peptide or protein disulfide bonds within a few minutes at room temperature. TCEP hydrochloride is unreactive toward other functional groups found in proteins. Unlike DTT (dithiothreitol), TCEP hydrochloride does not contain a free thiol, and therefore does not require removal before reaction with thiol-reactive reagents. TCEP reduces disulfides but apparently not mercury-thiol bonds. TCEP hydrochloride is compatible with many heavy atoms and may be used during heavy atom derivatization. TCEP hydrochloride is more stable at a higher pH and at higher temperatures than is DTT and for a longer period of time in buffers without metal chelators such as EGTA. With TCEP, removal of the reducing agent is not necessary prior to most applications, (e.g. histidine-tagged protein purification, protein crystallization).
TCEP is typically very soluble in aqueous buffers at nearly any pH. Therefore, working concentrations and 10X stock solutions may be readily prepared in most aqueous buffers. TCEP is stable in aqueous, acidic, and basic solutions. When TCEP is dissolved directly in water, the resulting pH is approximately 2.5. TCEP is not very stable in phosphate buffers, especially at neutral pH. Therefore, if TCEP is to be used in PBS buffers, prepare the working solution immediately before use. TCEP may be used as a substitute loading buffer for SDS-PAGE; use a final concentration of 50 mM TCEP. Since TCEP is charged in solution, it is not compatible for use in isoelectric focusing.
A 10 mM TCEP hydrochloride concentration can reduce the pH of a 100 mM buffer by approximately 0.5 pH units; 1 mM TCEP hydrochloride concentration reducing the pH of a 100 mM buffer by 0.05 pH units.
Stability in Solution
TCEP is stable in aqueous acidic and basic solutions. When TCEP is dissolved directly in water the resulting pH is 2.5. Various buffers may be used for the reductions. Studies indicate that no change in concentration occurs after 24 hour incubation at room temperature in 100 mM HCL, 100 mM NaOH, or any of the following 50 mM buffers Tris HCl (pH 7.5, 8.5, 9.5), HEPES(pH 6.8, 8.2), Borate(pH 8.2, 10.2), and CAPS (pH 9.7, 11.1). Even after weeks in these buffers, less than 20% of the TCEP was oxidized.
TCEP is not particularly stable in phosphate buffers, especially at neutral pH. Experiments indicate that TCEP completely oxidizes within 72 hours in 0.35M phosphate-buffered saline (PBS), pH 7.0. Approximately 50% oxidation occurs in the same amount of time in 0.15M PBS, pH 8.0. Only minimal oxidation occurs in PBS at pH > 10.5 or < 6.0. Therefore, if TCEP is to be used in PBS buffers, prepare the working solution immediately before use.
Solid TCEP hydrochloride is supplied packaged in argon to minimize oxidation.
Molecular formula C9H15O6P • HCl
Molecular weight 286.65
CAS [51805-45-9]
Beilstein Registry Number 3724376
Purity ≥99.0%
Stable at room temperature for up to 2 years after receipt.
TCEP盐酸盐
应用
- 结晶试验用稳定还原剂
特征
- 高可溶性
- 无臭非挥发性
- 在酸性和碱性pH下有效
描述
盐酸TCEP为三(2-羧乙基)膦盐酸盐.
盐酸TCEP是一种无臭、非挥发性的还原剂,比DTT或2-巯基乙醇更稳定有效.与DTT不同,盐酸TCEP在酸性PHS(PH 5)和PHS高于7.5时仍保持其还原能力,且在空气中稳定。盐酸TCEP可溶于水至每升310克。在大多数应用中,5-50毫米TCEP提供了足够的摩尔过量,可以在室温下几分钟内有效地减少肽或蛋白质二硫键。盐酸TCEP对蛋白质中的其他官能团没有反应。与DTT(二硫苏糖醇)不同,TCEP盐酸盐不含游离硫醇,因此在与硫醇反应试剂反应前不需要去除。TCEP减少了二硫键,但明显没有汞-硫醇键。盐酸TCEP与许多重原子相容,可用于重原子衍生。与DTT相比,盐酸TCEP在较高的pH值和较高的温度下更稳定,且在没有EGTA等金属螯合剂的缓冲液中具有更长的稳定性。使用TCEP时,在大多数应用之前不需要去除还原剂(例如组氨酸标记的蛋白质纯化、蛋白质结晶)。
TCEP通常在几乎任何pH值下都能溶于水缓冲液中。因此,工作浓度和10X储存溶液可以很容易地在大多数水缓冲器中制备。TCEP在水溶液、酸性和碱性溶液中是稳定的。当TCEP直接溶于水中时,其pH值约为2.5。TCEP在磷酸盐缓冲液中不太稳定,特别是在中性pH条件下。因此,如果要在PBS缓冲器中使用TCEP,请在使用前立即准备工作溶液。TCEP可作为SDS-PAGE的替代加载缓冲液;最终浓度为50 mm TCEP。由于TCEP是在溶液中充电的,所以它不适合用于等电聚焦。
10 mmTCEP盐酸盐浓度可使100 mm缓冲液的pH降低约0.5个pH单位;1mm TCEP盐酸TCEP浓度可使100 mm缓冲液的pH降低0.05个pH单位。
溶液稳定性
TCEP在酸性和碱性水溶液中是稳定的。当TCEP直接溶于水中时,其pH值为2.5。各种缓冲器可用于减缩。研究表明,在100 mm HCL、100 mm NaOH或以下任何50 mm缓冲介质Tris HCl(pH 7.5、8.5、9.5)、HEPES(pH 6.8、8.2)、硼酸盐(pH 8.2、10.2)和CAPS(pH 9.7,11.1)中,室温孵育24小时后,浓度不会发生变化。即使在这些缓冲的几个星期后,只有不到20%的TCEP被氧化。
TCEP在磷酸盐缓冲液中不特别稳定,特别是在中性pH条件下。实验表明,在pH7.0的0.35m磷酸盐缓冲溶液(PBS)中,TCEP在72h内完全氧化。约50%的氧化发生在相同的时间内,在0.15mPBS,pH8.0。PH>10.5或<6.0时,PBS只发生极少量的氧化。因此,如果要在PBS缓冲器中使用TCEP,请在使用前立即准备工作溶液。
固体TCEP盐酸盐被包装在氩气中,以减少氧化。
分子式C9H15O6P·HCl
分子量286.65
核证机关[51805-45-9]
伯尔斯坦登记处编号3724376
纯度≥99.0%
在室温下稳定,在收到后长达2年。
| HR2-651 | TCEP hydrochloride | 1 gram |
| HR2-801 | TCEP hydrochloride | 10 grams |