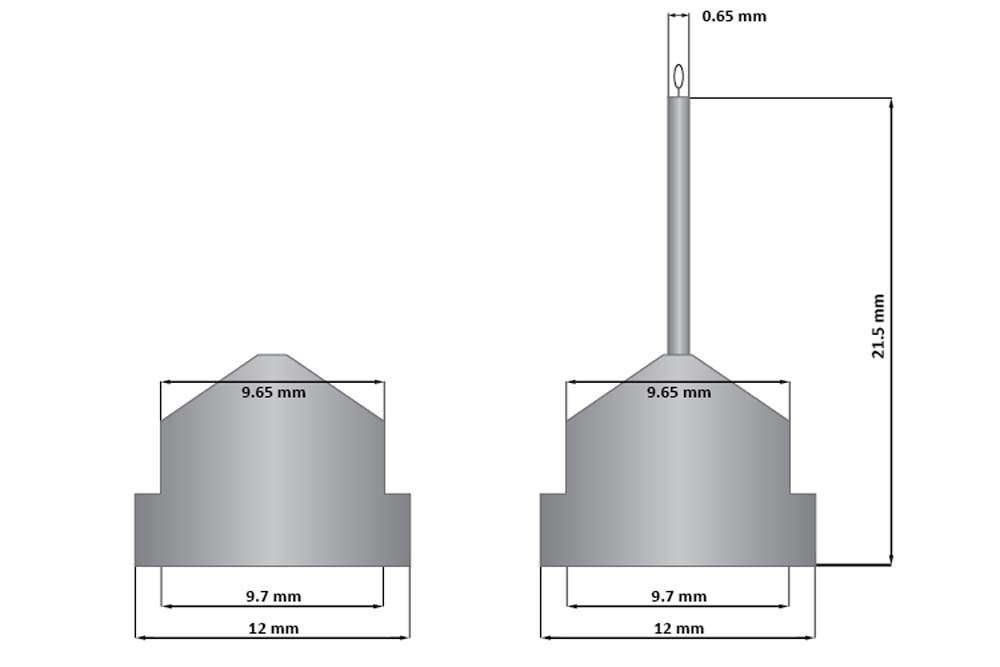
Hampton 带磁力结晶环(ALS型)底座 CrystalCap™ ALS HT
Hampton蛋白结晶试剂耗材
Hampton Research代理
欢迎访问Hampton Research官网或者咨询我们获取更多有关蛋白结晶试剂耗材产品信息。

APPLICATIONS
- Cryocrystallography
FEATURES
- ALS style crystal mount for cryocrystallography
- CrystalCap ALS HT attaches magnetically to CrystalCap Vial and Magnetic Goniometer Base
- Ledge free conical shape compatible with ALS style grippers, auto mounters and sample handlers
- 2D alphanumeric coding for sample tracking & management
- Color coded CryoLoop size
DESCRIPTION
The CrystalCap ALS HT is a magnetic sample mount (also known as a cap, pin or goniometer base) designed for cryocrystallography systems that accept ALS style sample mounts. The CrystalCap ALS HT attaches to a magnetic CrystalCap Vial and magnetic goniometer base. The tip of the CrystalCap accepts a Mounted CryoLoop™ or MicroTube™ fitted with a CryoLoop™.
The CrystalCap Vial is a 1.8 ml cryo vial featuring two small vents. A ring magnet is molded into the open end of the vial so that when the cap is positioned in the vial, the ring magnet holds the cap on the vial during cryogenic storage. The HR4-904 CrystalCap Vial does not have a magnet on the bottom of the vial.
The ledge free conical shape of the CrystalCap ALS HT is compatible with ALS style grippers, auto mounters and sample handlers.
The CrystalCap ALS HT features a two dimensional (2D) alphanumeric 16 x 16 data matrix code on the underside of the cap. Each cap is also color coded for CryoLoop size.
Note: CrystalCap ALS HT with Mounted CryoLoops are sold without vials. Vials HR4-904 sold separately.
Color Coded Cap…………CryoLoop Size
Red…………………………….0.025-0.05 mm
Green…………………………….0.05-0.1 mm
Blue………………………………..0.2-0.3 mm
Blue/Red………………………….0.3-0.4 mm
Green/Red………………………..0.4-0.5 mm
Yellow/Green……………………..0.7-1.0 mm
Compatible with the following Synchrotron Radiation Beamlines
North & South America
• The Advanced Light Source, Berkeley, California ALS 4.2.2, ALS 5.0.1, ALS 5.0.2, ALS 5.0.3, ALS 8.2.1, ALS 8.3.1, ALS 11.3.1, ALS 12.2.2, ALS 12.3.2, ALS 12.3.1-APX
• The Advanced Photon Source, Argonne, Illinois APS 14-BM-C BioCARS, APS 14-ID-B BioCARS, APS 17-ID IMCA-CAT, APS 19-BM, APS 19-ID, APS 22-BM SER-CAT, APS 22-ID SER-CAT, PS 23-BM-B GA/CA, APS 23-ID-B GA/CA, APS 23-ID-D GA/CA, APS 24-ID-C NE-CAT, APS 24-ID-E NE-CAT, APS 31-ID LR-CAT
• Center for Advanced Microstructures and Devices, Baton Rouge, Louisiana CAMD GCPCC
• Cornell High Energy, Synchrotron Source, Ithaca, New York CHESS A1, CHESS F1
• Canadian Light Source, Saskatchewan, Canada, CLS 08ID-1, CLS 08B1-1
• The Brazilian Synchrotron, Light Laboratory, Sao Paulo, Brazil LNLS D03B-MX1, LNLS W01B-MX2
• Stanford Synchrotron Radiation Laboratory, Menlo Park, California SSRL BL7-1, SSRL BL9-1, SSRL BL9-2, SSRL BL11-1, SSRL BL12-2, SSRL BL14-1
Europe
• Kurchatov Center for Synchrotron Radiation and Nanotechnology, Moscow, Russia KCSRNT K4.4
• Max-Lab, Lund University, Sweden MAX II I711, MAX II I911-2, MAX II I911-3
• MPG/DESY, Hamburg, Germany MPGDESY BW6
• SOLEIL, Saint-Aubin, France SOLEIL PROXIMA1, SOLEIL PROXIMA2
Asia & Australia
• Shanghai Synchrotron Radiation Facility, Shanghai, China SSRF BL17U1
• National Synchrotron Radiation Research Center, Taiwan NSRRC BL13B1, NSRRC BL13C1
• Pohang Accelerator Laboratory, Pohang, South Korea PAL 2D, PAL 5C, PAL 7A
• Photon Factory, Tsukuba, Japan PF BL-5A, PF BL-17A, PF AR-NW12A
• Super Photon ring-8 GeV, Japan SPRING-8 BL12B2, SPRING-8 BL24XU, SPRING-8 BL26B1, SPRING-8 BL26B2, SPRING-8 BL32B2, SPRING-8 BL32XU, SPRING-8 BL38B1, SPRING-8 BL41XU, SPRING-8 BL44B2, SPRING-8 BL44XU
结晶帽™ALS HT
应用
- 冰晶术
特征
- AlS型结晶架
- 结晶帽ALS HT磁性附着在结晶帽Vial磁力仪基座
- 可与ALS风格的夹持器、自动装配器和示例处理程序兼容的自由锥形形状
- 用于样本跟踪和管理的二维字母数字编码
- 彩色编码冷冻环尺寸
描述
结晶帽ALS HT是一个磁性样品安装(也称为盖子,引脚或测角器底座),为接受ALS型样品安装的冰晶系统设计。结晶帽ALS HT附着在一个磁晶帽和磁测角仪的基座上。水晶帽的顶端接受安装的冷冻环™。
水晶瓶盖是一个1.8毫升的冰瓶,有两个小通风口。将环形磁铁模压到瓶的开口端,以便当盖被放置在瓶中时,环磁铁在低温储存期间将盖保持在瓶上。HR4-904水晶帽瓶底部没有磁铁。
水晶帽ALS HT的无棱镜锥形形状与ALS风格的夹持器、自动装配机和样品处理程序兼容。
水晶帽ALS HT的特点是一个二维字母数字16×16数据矩阵代码在帽子的底部。每个帽子的颜色也编码为Cryoloop大小。
注:水晶帽ALS HT与安装超低温是出售没有小瓶。小瓶HR4-904单独出售.
颜色编码,第.章,冷冻环尺寸
Red..0.025-0.05 mm
Green..0.05-0.1 mm
Blue..0.2-0.3 mm
Blue/Red..0.3-0.4 mm
绿/红.0.4-0.5毫米
黄/绿.0.7至1.0毫米
与下列同步辐射光束线兼容
北美和南美洲
·高级光源,加州伯克利,ALS 4.2.2,ALS 5.0.1,ALS 5.0.2,ALS 5.0.3,ALS 8.2.1,ALS 8.3.1,ALS 11.3.1,ALS 12.2.2,ALS 12.3.2,ALS 12.3.1-APX
*高级光子源,阿贡,伊利诺斯州APS 14-BM-C BioCARS,APS 14-ID-B BioCARS,APS 17-ID IMCA-CAT,APS 19-BM,APS 19-ID,APS 22-BM SER-CAT,APS 22-ID SER-CAT,PS 23-BM-B GA/CA,APS 23-ID-B GA/CA,APS 23-ID-D GA/CA,APS 24-ID-C NE-CAT,APS 24 ID-E NE-CAT,APS 31-ID LR-CAT
·先进微结构和器件中心,巴吞鲁日,路易斯安那州CAMD GCPCC
·康奈尔高能,同步加速器源,伊萨卡,纽约国际象棋A1,国际象棋F1
加拿大光源,加拿大萨斯喀彻温省,CLS 08ID-1,CLS 08B1-1
*巴西同步加速器,光实验室,巴西圣保罗,LNLS D03B-MX1,LNLS W01B-MX2
·斯坦福同步辐射实验室,加州门罗公园,SSRL BL 7-1,SSRL BL 9-1,SSRL BL 9-2,SSRL BL 11-1,SSRL BL 12-2,SSRL BL 14-1
欧洲
·库尔恰托夫同步辐射和纳米技术中心,俄罗斯莫斯科KCSRNT K4.4
·瑞典隆德大学实验室,Max II I 711,Max II I 911-2,Max II I 911-3
*MPG/DESY,德国汉堡,MPGDESY BW6
太阳、圣奥宾、法国太阳PROXIMA 1、太阳PROXIMA 2
亚洲和澳大利亚
·上海同步辐射装置,中国上海SSRF BL17U1
·国家同步辐射研究中心,台湾NSRRC BL13B1,NSRRC BL13C1
·波航加速器实验室,韩国POHANG,PAL 2D,PAL 5C,PAL 7A
·光子工厂,筑波,日本PF BL-5A,PF BL-17A,PF AR-NW12A
超级光子环-8 GeV,日本Spring-8 BL12B2,Spring-8 BL24XU,Spring-8 BL26B1,Spring-8 BL26B2,Spring-8 BL32B2,Spring-8 BL32XU,Spring-8 BL38B1,Spring-8 BL41XU,Spring-8 BL44B2,Spring-8 BL44XU
| HR8-200 | CrystalCap ALS HT | 0.025-0.05 mm CryoLoop – 30 pack |
| HR8-202 | CrystalCap ALS HT | 0.05-0.1 mm CryoLoop – 30 pack |
| HR8-204 | CrystalCap ALS HT | 0.1-0.2 mm CryoLoop – 30 pack |
| HR8-206 | CrystalCap ALS HT | 0.2-0.3 mm CryoLoop – 30 pack |
| HR8-208 | CrystalCap ALS HT | 0.3-0.4 mm CryoLoop – 30 pack |






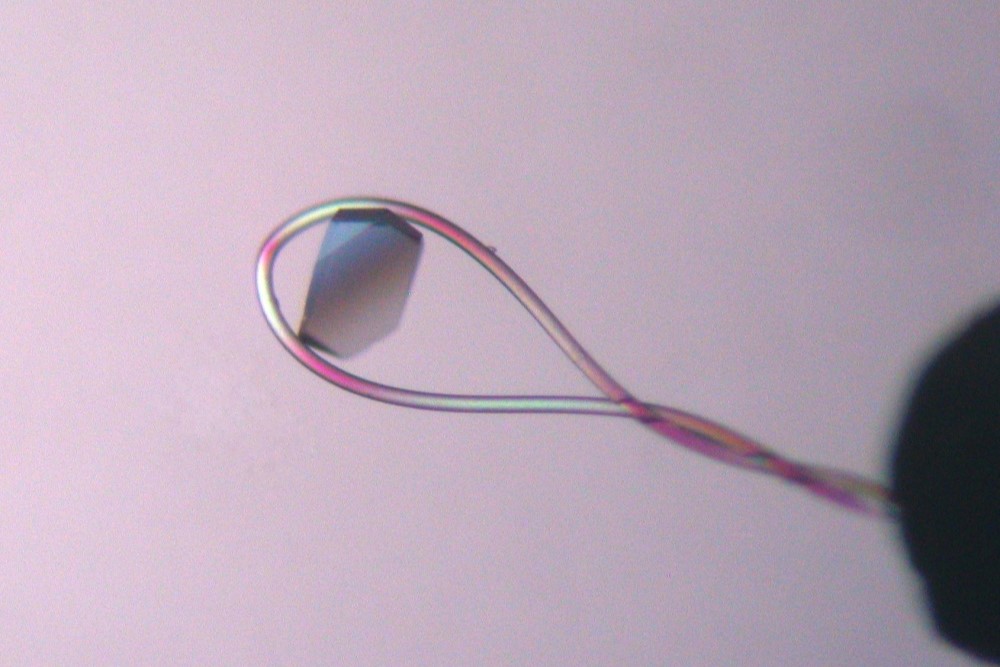
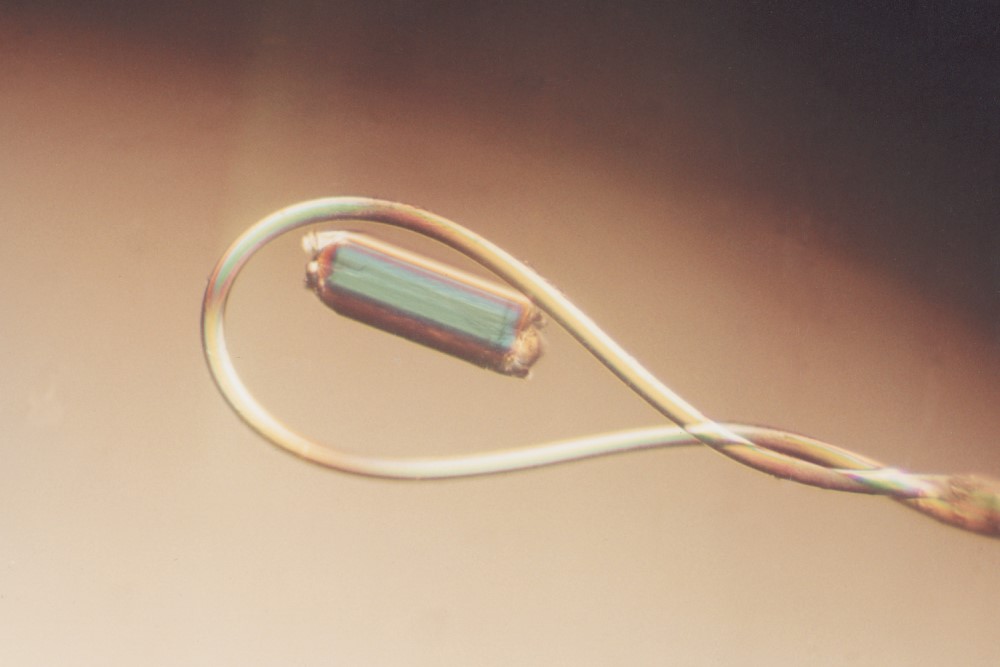

 X-ray data collection
X-ray data collection